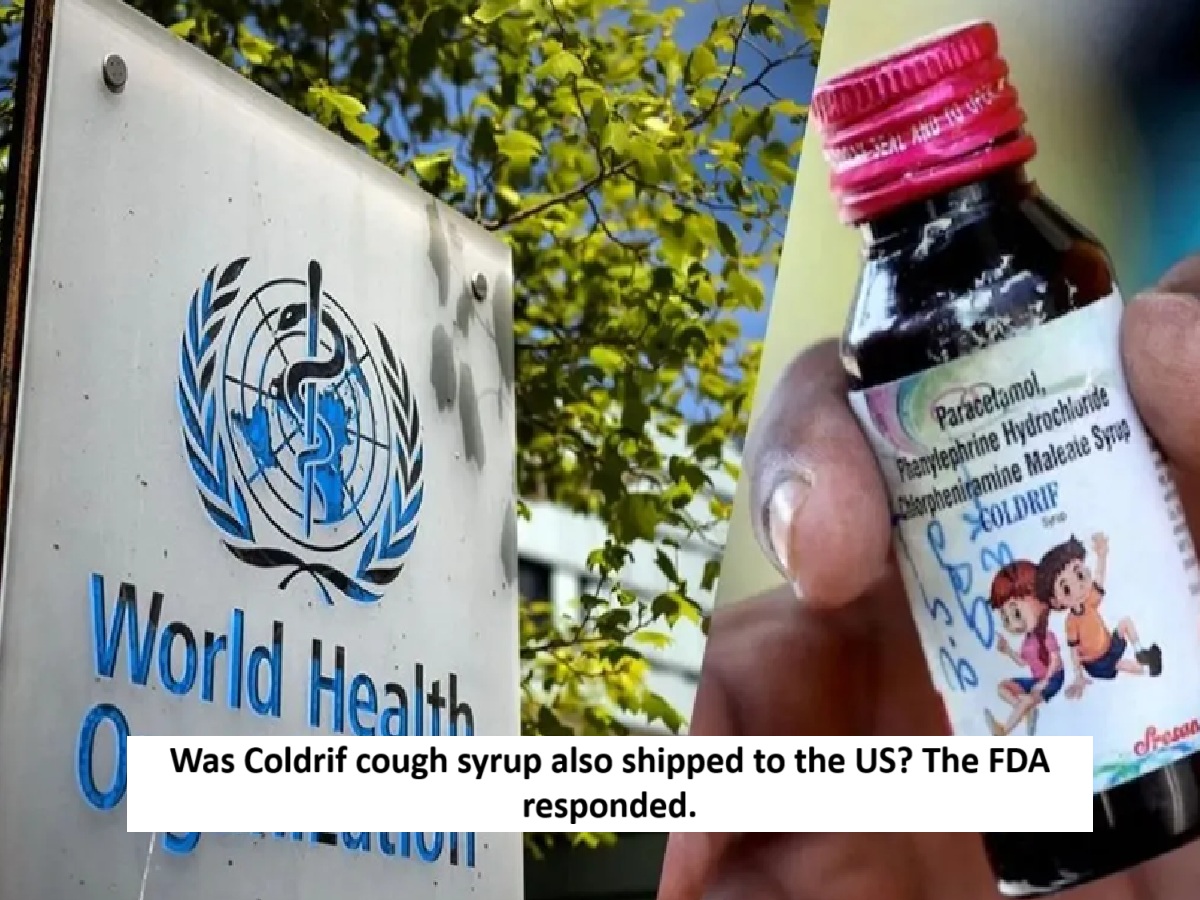
News Topical, Digital Desk : Children died from toxic cough syrup in Madhya Pradesh and Rajasthan. Following the incident, the World Health Organization (WHO) on Wednesday asked India for clarification on whether these medicines were exported to other countries. Following this, the US Food and Drug Administration clarified that the toxic cough syrup linked to the deaths of children in India was not shipped to the United States.
In fact, the World Health Organization has stated that there are "regulatory deficiencies" in the testing of cough syrups sold locally in India. The World Health Organization has sought clarification from India on whether the cough syrup linked to the deaths of children in the country was exported to other countries as part of a regular process.
Toxic cough syrups not shipped to the US
The US Food and Drug Administration confirmed on Friday that toxic cough syrups linked to the deaths of children in India have not been shipped to the US. The US FDA said it is aware of reports of toxicity caused by diethylene glycol and ethylene glycol in children's cough and cold medicines in India.
FDA alert before departure to the US
The FDA said that India's health authority, the Central Drugs Standard Control Organization, has informed the US regulator that these products are not being exported from India to any other country. The FDA also stated that it is vigilant to prevent such toxic drugs from entering the US and has urged manufacturers to ensure that medicines sold in the US are safe and of the highest quality.
According to officials, children in India have died in the past month after consuming cough syrup containing toxic diethylene glycol, which was found to contain diethylene glycol and ethylene glycol in levels exceeding the permissible limits, similar to those found in Coldrif cough syrup. Indian authorities have advised people to avoid Coldrif cough syrup and two other brands.
Read More: Indian influencer mistreated in South Korea, Indian Embassy issues advisory; What are the rules?
--Advertisement--

 Share
Share



